The Ultimate Guide to Low Dose Naltrexone: A Comprehensive Approach to Pain, Autoimmune Conditions, and More for Michigan Patients

What is Low Dose Naltrexone (LDN)?
Low Dose Naltrexone (LDN) is increasingly being recognized for its potential role in managing chronic pain, autoimmune disorders, and inflammatory conditions. While naltrexone was originally approved for addressing opioid addiction, lower doses (typically 0.5 mg – 4.5 mg) have been explored for their immunomodulatory and analgesic properties.
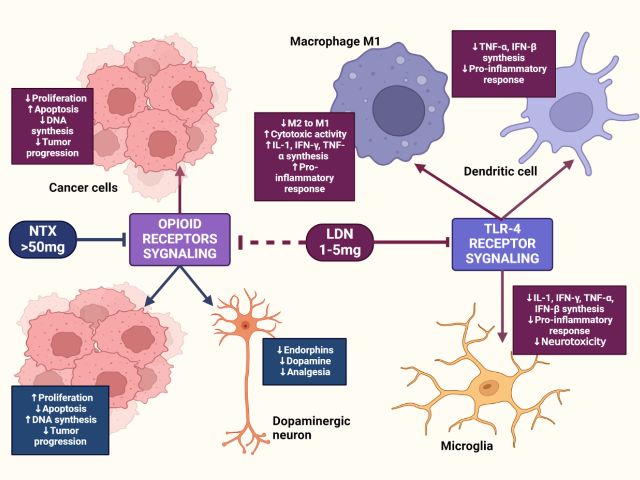
Naltrexone, an opioid receptor antagonist, was approved by the FDA in 1984 for addressing opioid addiction at doses of 50 to 100 mg. In the 1980s, Dr. Bernard Bihari discovered that significantly lower doses (1 to 5 mg) could modulate the immune system, leading to the concept of Low Dose Naltrexone (LDN). This innovation opened new avenues for addressing conditions beyond addiction, particularly those involving immune dysregulation.
LDN: Mechanism of Action
LDN functions as a transient opioid receptor antagonist. At low doses, it temporarily blocks opioid receptors, leading to a compensatory upregulation of endogenous opioids—including beta-endorphins and Met-enkephalin (opioid growth factor, OGF) — which play a role in immune regulation, pain modulation, and inflammation control.
Additionally, research suggests LDN’s non-opioid pathways contribute to its anti-inflammatory effects by inhibiting Toll-like receptor 4 (TLR4) on microglia. This reduces the release of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which are implicated in chronic pain and autoimmune pathology.
Why LDN Has Become So Popular with Prescribers
LDN’s popularity stems from its potential to offer therapeutic benefits with minimal side effects. Its ability to modulate the immune system and reduce inflammation makes it an attractive option for patients seeking alternatives to conventional therapies that may have failed for them, especially for chronic conditions that are resistant to standard therapies.
The Versatility of LDN
LDN has been explored by many of Michigan’s top practitioners and prescribed for autoimmune diseases, chronic pain syndromes, neurodegenerative disorders, and even certain cancers. This broad applicability is due to its immunomodulatory and anti-inflammatory properties, which address the underlying mechanisms of various diseases.
Clinical Applications of LDN
Emerging evidence supports LDN’s potential therapeutic role in several conditions, including:
- Chronic Pain Syndromes: Fibromyalgia, neuropathic pain, migraines.
- Autoimmune Disorders: Multiple sclerosis, Crohn’s disease, rheumatoid arthritis, Hashimoto’s thyroiditis.
- Inflammatory Conditions: Irritable bowel syndrome, interstitial cystitis, chronic fatigue syndrome.
- Mood Disorders: Some studies suggest LDN may have neuroprotective benefits in depression and PTSD.
- Oncology Support: Preclinical studies indicate that LDN, via OGF upregulation, may exhibit anti-proliferative effects in certain malignancies.
Practitioners Who Prescribe LDN
A diverse group of healthcare providers prescribe LDN, including physicians, nurse practitioners, and physician assistants. These practitioners may come from various specialties such as rheumatology, neurology, pain management, and integrative medicine. The decision to prescribe LDN often depends on the provider’s familiarity with its off-label uses and the specific needs of the patient. Expert LDN pharmacists at Healthway Compounding Pharmacy guide practitioners through the process of prescribing. This offers patients more, with compounded options that may elevate their experience and health outcomes.
Why LDN Requires Compounding
Commercial naltrexone is only available in 50-mg tablets — far exceeding the doses used in LDN therapy. To achieve precise dosing, LDN must be compounded into formulations appropriate for patient-specific needs, including:
- Capsules: Precise, customized dosing.
- Oral Liquid: Flexible titration for dose adjustments.
- Sublingual Drops: Enhanced absorption for patients with GI concerns.
- Topical Cream: Non-oral alternative for select conditions.
- Sublingual Lozenge/Troche: Convenient, buccal absorption with slower dissolution.
- Nasal Spray: Rapid absorption through the nasal mucosa for patients with swallowing issues or absorption concerns.
- Topical Nail: Targeted delivery for nail-specific conditions like lichen planus or psoriasis.
- Ear Drops: Localized treatment option for inflammatory ear conditions.
- Vaginal Cream: Local administration for gynecologic autoimmune or inflammatory conditions.
Integrating LDN into Patient Care
LDN’s low risk of adverse effects and emerging clinical evidence make it an option worth considering for patients with treatment-resistant pain, inflammatory conditions, or autoimmune disorders. Given its unique mechanism, LDN is often used adjunctively with other therapies rather than as a monotherapy.
Titration protocols vary, but many prescribers initiate therapy at 1.5 mg – 3 mg and adjust based on patient response. As with all compounded therapies, collaboration with an experienced compounding pharmacy is essential to ensure formulation consistency and bioavailability.
Scholarly Articles Supporting LDN Use
Several peer-reviewed studies have explored LDN’s therapeutic potential:
Patten, D. K., Schultz, B. G., & Berlau, D. J. (2018). The safety and efficacy of low‐dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn’s disease, and other chronic pain disorders. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 38(3), 382-389.
Low-dose naltrexone for disease prevention and quality of life
Brown, N., & Panksepp, J. (2009). Low-dose naltrexone for disease prevention and quality of life. Medical hypotheses, 72(3), 333-337.
Preclinical and clinical studies into the bioactivity of low-dose naltrexone (LDN) for oncotherapy
Qu, N., Meng, Y., Handley, M. K., Wang, C., & Shan, F. (2021). Preclinical and clinical studies into the bioactivity of low-dose naltrexone (LDN) for oncotherapy. International Immunopharmacology, 96, 107714.
The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain
Younger, J., Parkitny, L., & McLain, D. (2014). The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clinical rheumatology, 33, 451-459.
Lie, M. R., van der Giessen, J., Fuhler, G. M., de Lima, A., Peppelenbosch, M. P., van der Ent, C., & van der Woude, C. J. (2018). Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. Journal of translational medicine, 16, 1-11.
These are just a few of many peer-reviewed literature that supports LDN’s potential in several health conditions.
What Sets Healthway Compounding Pharmacy Apart
Healthway Compounding Pharmacy, located in Saginaw, Michigan, has been a pioneer in compounding specialty medications since 1985. As the first pharmacy in Michigan to achieve PCAB accreditation in 2007, Healthway has consistently maintained this standard of excellence.

Our commitment to personalized medicine ensures patients receive LDN formulations tailored to their specific needs, adhering to the highest quality and safety standards. Serving regions north of Saginaw, including the Upper Peninsula and Thumb area, we are dedicated to improving patient outcomes through innovative compounded medications. We are able to ship state-wide with a valid prescription.
If you are considering incorporating LDN into your practice, consult with our compounding pharmacists to discuss formulation options tailored to your patients’ needs.
If you’re a patient interested in LDN, ask your healthcare provider to consider reaching out to our team.
References
Brown, N., & Panksepp, J. (2009). Low-dose naltrexone for disease prevention and quality of life. Medical hypotheses, 72(3), 333-337. Link
Bruun, K. D., Amris, K., Vaegter, H. B., Blichfeldt-Eckhardt, M. R., Holsgaard-Larsen, A., Christensen, R., & Toft, P. (2021). Low-dose naltrexone for the treatment of fibromyalgia: protocol for a double-blind, randomized, placebo-controlled trial. Trials, 22, 1-13. Link
Ciwun, M., Tankiewicz-Kwedlo, A., & Pawlak, D. (2024). Low-dose naltrexone as an adjuvant in combined anticancer therapy. Cancers, 16(6), 1240. Link
Choubey, A., Girdhar, K., Kar, A. K., Kushwaha, S., Yadav, M. K., Ghosh, D., & Mondal, P. (2020). Low-dose naltrexone rescues inflammation and insulin resistance associated with hyperinsulinemia. Journal of Biological Chemistry, 295(48), 16359-16369. Link
Kim, P. S., & Fishman, M. A. (2020). Low-dose naltrexone for chronic pain: update and systemic review. Current pain and headache reports, 24(10), 64. Link
Lie, M. R., van der Giessen, J., Fuhler, G. M., de Lima, A., Peppelenbosch, M. P., van der Ent, C., & van der Woude, C. J. (2018). Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. Journal of translational medicine, 16, 1-11. Link
Patten, D. K., Schultz, B. G., & Berlau, D. J. (2018). The safety and efficacy of low‐dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn’s disease, and other chronic pain disorders. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 38(3), 382-389. Link
Qu, N., Meng, Y., Handley, M. K., Wang, C., & Shan, F. (2021). Preclinical and clinical studies into the bioactivity of low-dose naltrexone (LDN) for oncotherapy. International Immunopharmacology, 96, 107714. Link
Sudakin, D. (2016). Naltrexone: not just for opioids anymore. Journal of Medical Toxicology, 12, 71-75. Link
Trofimovitch, D., & Baumrucker, S. J. (2019). Pharmacology update: low-dose naltrexone as a possible nonopioid modality for some chronic, nonmalignant pain syndromes. American Journal of Hospice and Palliative Medicine®, 36(10), 907-912. Link
Younger, J., Parkitny, L., & McLain, D. (2014). The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clinical rheumatology, 33, 451-459. Link